Governance
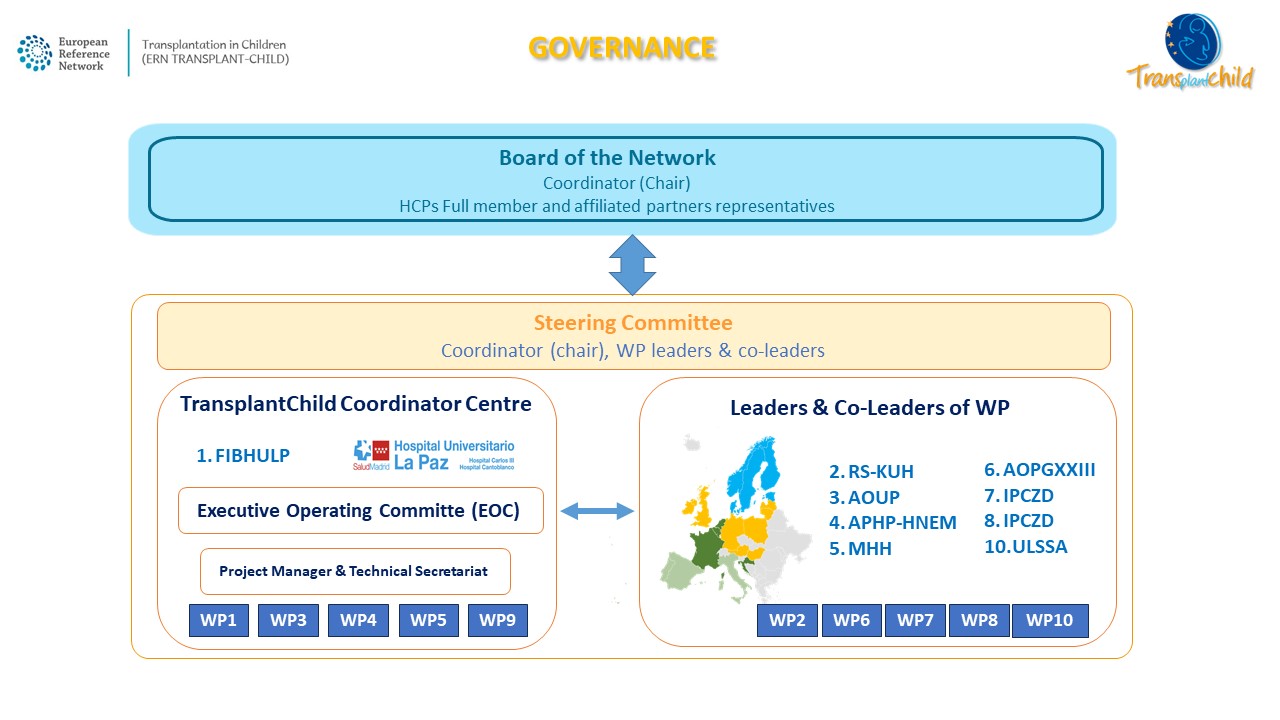
TransplantChild Executive Operating Committee
(EOC)

Dr. Francisco Hernández Oliveros
Coordinator of ERN TransplantChild

Dr. José Jonay Ojeda Feo
Co-Coordinator of ERN TransplantChild

Dr. Esteban Frauca Remacha
EOC WP4 Support

Dr. Álvaro González Rocafort
EOC WP6 Support

Dr. Yadira Bravo Gallego
EOC WP5 Support

Dr. Marta Melgosa Hijosa
EOC WP7 Support

María Jesús Pascau González-Garzón
EOC WP2 Support

Dr. Susana Noval Martín
Deputy Director of children’s hospital La Paz University Hospital

Dr. Javier Cobas Gamallo
CEO – Subdirector La Paz University Hospital
TransplantChild Technical Secretariat

Ana Merino Fernández
Project Manager

Marta Casales Santa
Project Manager

Dr. Yadira Bravo Gallego
Medical Advisor

Jorge Rioja Donoso
Communications and Analysis Manager

Miguel Clemente Bernal
eHealth Support Manager
Members of the network
Full Members
INVISIBLE
BELGIUM
- Cliniques Universitaires Saint-Luc
- Princess Elisabeth Children’s Hospital
DENMARK
- Odense University Hospital
- Copenhagen University Hospital Rigshospitalet
FRANCE
- Bicêtre Hospital
- Hôpital Necker-Enfants malades
FINLAND
- HUS Helsinki University Hospital
GERMANY
- Hannover Medical School
- University Medical Center Hamburg-Eppendorf
ITALY
- Azienda Ospedale- Università Padova
- Istituto Mediterraneo per i Trapianti e le Terapie ad alta specializzazione – ISMETT
- Ospedale Papa Giovanni XXIII
- Ospedale Pediátrico Bambino Gesù
- AOU Città della Salute e della Scienza di Torino
IRELAND
- Children’s Health Ireland
LITHUANIA
- Vilnius University Hospital Santariskiu Klinikos
NETHERLANDS
- UMC Utrecht
- Amsterdam University Medical Centres
- University Medical Center Rotterdam
NORWAY
- Oslo University Hospital
POLAND
- Children’s Memorial Health Institute
PORTUGAL
- Centro Hospitalar Universitário de Santo António
- Centro Hospitalar e Universitário de Coimbra
- Hospital Santa Maria. Centro. Hospitalar Lisboa Norte
SPAIN
- Hospital Universitario La Paz
- Hospital Universitario Virgen del Rocío
- Hospital del Niño Jesús
- Hospital General Universitario Gregorio Marañón
- Hospital Universitario Vall d’Hebron
- Hospital Sant Joan de Déu
SWEDEN
- Children’s Hospital, Skåne University Hospital
- Karolinska University Hospital
- Sahlgrenska Universitetssjukhuset
Affiliated Partners
INVISIBLE
AUSTRIA
- Centre for Pediatric Lung Transplantation, Medical University of Vienna
CROATIA
- University Hospital Centre Zagreb
ESTONIA
- Tartu University Hospital
HUNGARY
- Semmelweis University
LATVIA
- Children’s Clinical University Hospital, Riga
LUXEMBOURG
- Centre Hospitalier du Luxembourg
MALTA
- Mater Dei Hospital
Supporting Partners
INVISIBLE
UNITED KINGDOM
- King’s College Hospital NHS Foundation Trust
- Birmingham Women’s and Children’s NHS Foundation Trust
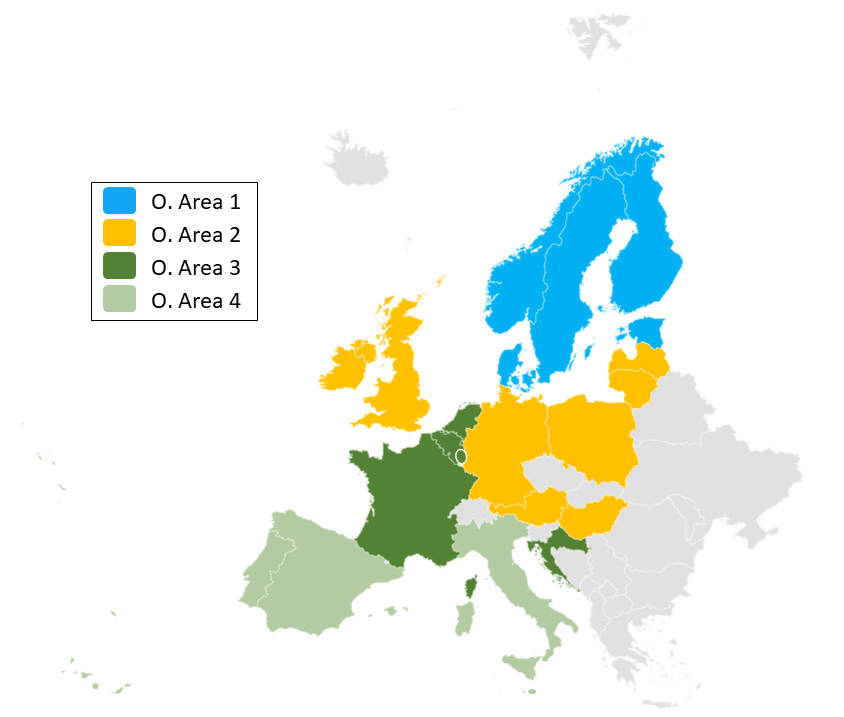
TransplantChild Operational Areas
Learn more about how the ERN is organized in Operational Areas
European activity on PT
Interactive Map of Paediatric Transplantation Activity in Europe
INVISIBLE
About Interactive Map
ERN Transplantchild wants to promote the visibility of information regarding pediatric transplantation by the creation of European pediatric transplantation map, in order to locate the specific experience, expertise, and knowledge in a field as wide as pediatric transplantation.
Objectives
The collected information about the paediatric transplantation activity across Europe during 2012-2016 enable us to define:
- Who we currently are (and what centers are currently not and should be in the network)
- To analyse the number and distribution of existing centers and transplantation programs
- To identify the equity in access to transplantation for pediatric candidates across Europe, and
- Extend the network to all EU member states and thus provide access to the network to all patients and families across the EU.
The Health Care working group through of multidisciplinary collaboration group decided to define the EU map of the paediatric transplantation activities, including both solid organ and hematopoietic stem cell transplants.
To achieve this goal, we kindly request the collaboration of all European National Agencies to provide paediatric donation and transplant information between the years 2012-2016. Information on transplanted patients under the age of 18 was collected including:
- The number of pediatric solid organ transplantations by type of organ (kidney, liver, lung, heart, and intestinal/multivisceral).
- The number of pediatric hematopoietic stem cell transplantations.
- The number of transplants (both solid organ and hematopoietic) per Hospitals of each country.
Participants
As agreed by all the attending Authorities at the 14th Meeting of the Competent Authorities held in Brussels (27-28th of June 2018), the ERN TransplantChild is putting together the paediatric transplantation in Europe to deliver a complete map of the activity and expertise. Until the end of December 2018, we have received a response from the following participants as representatives of their National agencies:
1. Bulgaria
Violetta Marinkova – Senior Expert – Bulgarian Executive Agency for Transplantation
2. Czech Republic
Prof.MUDr. Miloš Adamec, CSc – ředitel KST – Koordinační středisko transplantací
3. Estonia
Mrs. VIRGE PALL, MD – Director of Transplantation Centre – Tartu University Hospital
4. Greece
Hellenic Transplant Organization – Εθνικός Οργανισμός Μεταμοσχεύσεων
5. Ireland
Lynn Martin – National Organ Donor Co-Ordinator – Organ Donation and Transplant Ireland
6. Italy
Paola Di Ciaccio – Head of Foreign Affairs Division – Italian National Transplant Centre – Italian National Institute of Health
7. Lithuania
Vita Petronytė – Senior specialist – Transplant Coordination Department – National Transplant Bureau – Under the Ministry of Health of the Republic Lithuania
8. Poland
Jarosław Czerwiński – Centrum Organizacyjno-Koordynacyjne do Spraw Transplantacji – “Poltransplant”
9. Portugal
Catarina Bolotinha – Assessora da Coordenação Nacional da Transplantação – Serviços Centrais – Instituto Português de Sangue e da Transplantação, IP
10. Spain
MARINA ALVAREZ – Organización Nacional de Trasplantes – Ministerio de Sanidad, Consumo y Bienestar Social
11. Slovakia
Daniel Kuba, Magdaléna Krátka – Bratislava, Slovenská republika
12. Slovenia
Barbara Uštar – Zavod RS za presaditve organov in tkiv Slovenija-transplant – Ljubljana – Slovenija
13. United Kingdom
Rachel Hogg – ODT Statistical Enquiries – NHS -UK
14. Eurotransplant Austria, Belgium, Croatia, Denmark, Germany, Hungary, Netherlands, Switzerland
Jacob de Boer, MD – Medical Staff ET – Eurotransplant International Foundation – Josephine Wadewitz – Deutsche Stiftung – Organtransplantation – Germany
15. Scandiatransplant Denmark, Finland, Iceland, Norway, Sweden Ilse Duus Weinreich
Clinical Data and Office Manager – Scandiatransplant – Aarhus University Hospital, Skejby – Helle Thiesson – OUH – Odense Universitetshospital – Svendborg Sygehus
Activity data collection
The information was required in a determined format, and each agency should be structured Activity data according to the follow instructions:
- Divided by hospitals.
- Separated by age: one group for patients younger than 16 years old, and a second one for those between 16 and 18 years old.
- Type of transplant: HSCT, liver, kidney, heart, lung and Intestinal/multivisceral.
- Years range from 2012 to 2016.
- As an optional, for liver and kidney the data were discriminated by by living or cadaveric (deceased) donor. In the case of HSCT, the data were divided by autologous or allogeneic transplant. The Health Care working group through of multidisciplinary collaboration group decided to define the EU map of the paediatric transplantation activities , including both solid organ and hematopoietic stem cell transplants. In order to achieve the highest possible reliability of the data these have been requested through all possible available ways like the national agencies for organ donation and allocation, European transplantation scientific societies, and center own registries . The map was prepared by the WG and was reviewed and approved by both the Group Coordinator and the Technical Director. The final version will be distributed online and the analysis of the results wil be discussed by the working group to obtain the mainly conclusions aligned to the objectives of this activity.
Acknowledges
We wish to acknowledge the support of the Unit B4 of Medical Products: Quality, Safety and Innovation of the DG Health & Food Safety (SANTE) for their invitation to the 14th Meeting of the Competent Authorities on Organ Donation and Transplantation. Without their endorsement and the generous collaboration of the representatives of each of the European Competent Authorities on Organ Donation and Transplantation, this work would have not been possible.
Allocation of paediatric transplants in Europe
Allocation of paediatric transplants in Europe (0-16)
Allocation of paediatric transplants in Europe (17-18)
Total activity in Europe (2012-2016)
Projects
Joint Action JARDIN
More
JARDIN is a Joint Action created for the Integration of ERNs into the
National Health Systems of the EU Member States, plus Norway and
Ukraine.
JARDIN’s mission is to improve the accessibility of ERNs for people
living with rare diseases or complex conditions, through
recommendations and pilot projects for implementation in key areas of
action, such as patient pathways, national reference networks and data
management for rare diseases.
European Rare Disease Research Coordination and Support Action
More
GA number: 964908
The aim of the European Rare Disease Research Coordination and Support Action consortium (ERICA), in which all 24 European Reference Networks (ERNs) take part, is to build on the strength of the individual ERNs and create a platform that integrates all ERNs research and innovation capacity.
European Rare Diseases Research Alliance
More
To leave no one behind, over 170 organisations championed by the European Union and members states are working hand in hand to make Europe a world leader in rare diseases research and innovation. The European Rare Disease Research Alliance (ERDERA) takes over EJPRD to deliver concrete health benefits to rare disease patients in the next decade by advancing prevention, diagnosis and treatment research.
PaEdiatric Transplantation European Registry “PETER”
More
GA number: 947629
The PaEdiatric Transplantation European Registry (PETER) will be a platform focused on paediatric transplantation within the EU, including all types of transplantations (i.e., both solid organ transplantation –SOT- and haematopoietic stem cell transplantations-HSTC-). This platform integrates information from clinicians and patients into research projects.





